RNI Consulting supports you in your international export projects.
The regulatory aspects of a project should be anticipated ahead of product development in order to:
Integrate an international marketing extension strategy from the beginning
A nutrition product with health benefits may fall underdifferent regulatory statuses across countries:
- A food supplement (« Food Supplement ») in Europe, a dietary supplement in the US, a Natural Health Product in Canada, therapeutic goods in Australia…
- A beverage with addition of nutrients and/or added value substances can be classified as an enriched food, a food supplement, a health product…
Develop a product in accordance with:
- The applicable regulation of the targeted countries,
- The targeted health and nutrition benefits with the choice of active ingredients in compliance with regulation.
Consider new marketing opportunities through an inventory of the regulation applicable to the product;
- Cost, deadlines and requirementsof the registration process will impact the choice of countries for market.
Avoid any non-compliance at the time of marketing,
Assist with marketing goals by offering regulatory expertise as well as the scientific substantiation required for desired claims
Our solutions for international development:
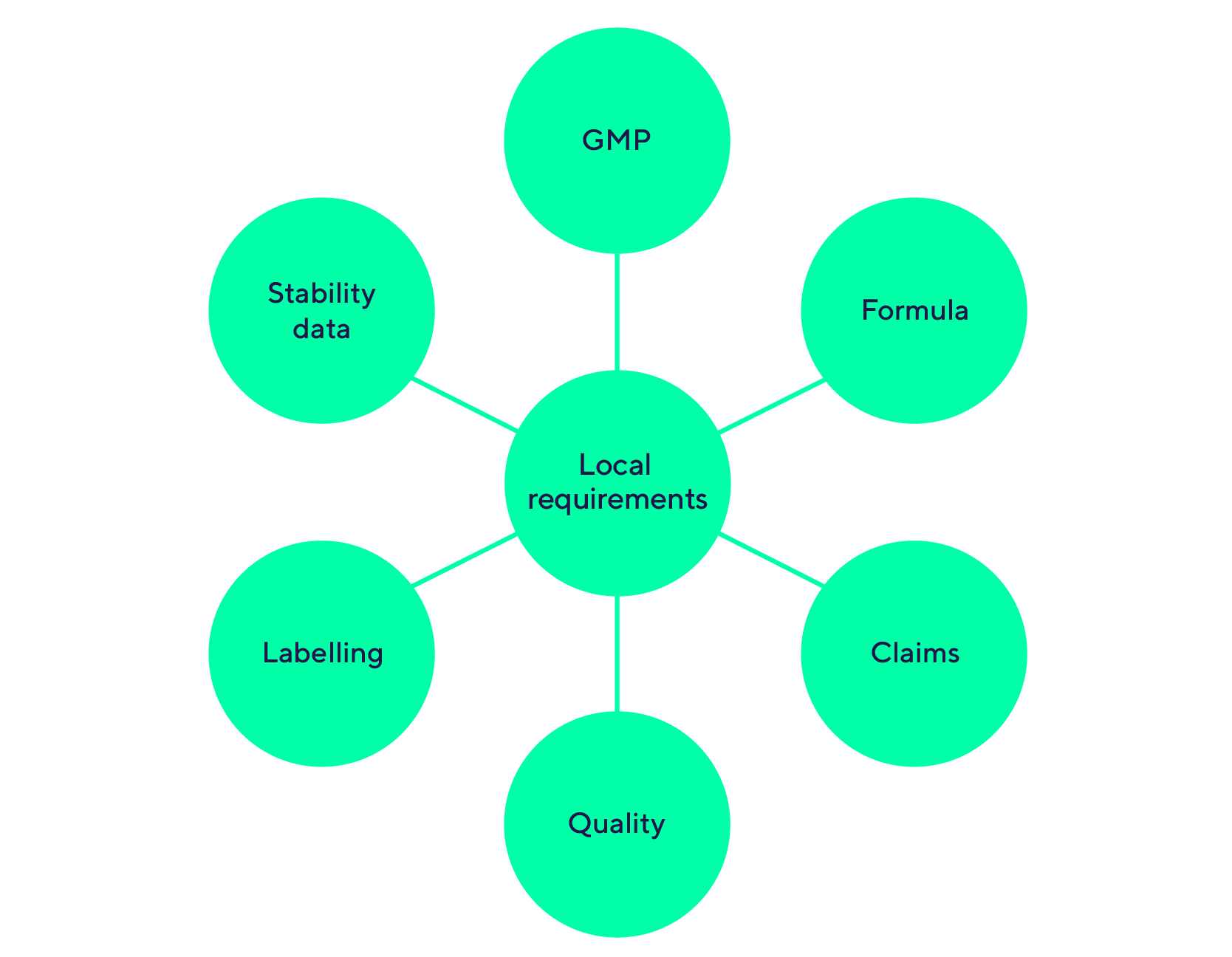
Gap analysis of your data according to local/regional/international requirement :
Communication and advertising assessment (labelling / websites /TV / flyers…)
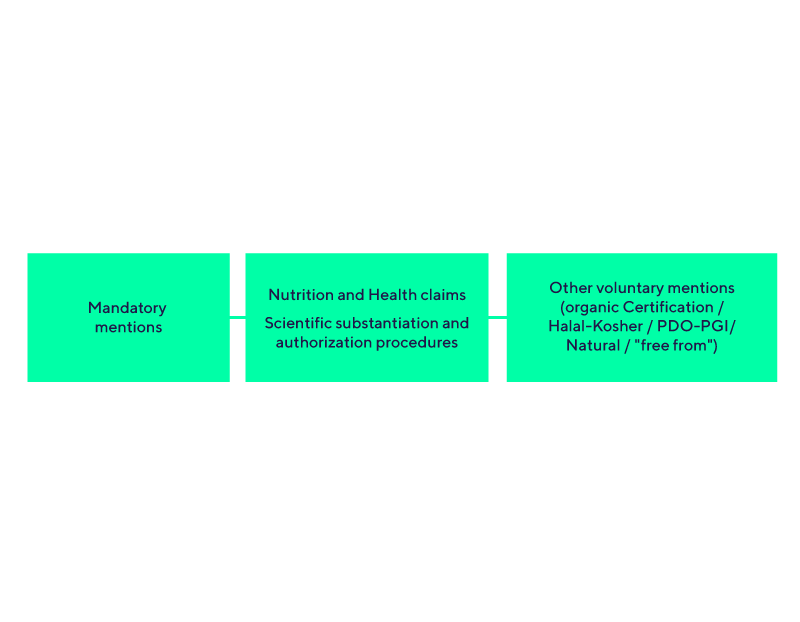
Strategic marketing authorization procedures
- Depending on the status, delays, costs, data requirements, markets.
- New ingredients (NDIN, GRAS, Novel Food) – Borderline products (cosmetic, health food, Medical Devices), product registrations.
Regulatory strategy for quick access to market and quick win products
Major discussion with the authorities (EC/FDA/National authorities) and the associations and main partners in the different countries:
- Status of an ingredient: food vs drug vs new ingredient
- Status of a product depending on its composition, presentation and communication
- Status of a claim: health claim, structure function claim, therapeutic claim, beauty claim
- Regulation of the additives worldwide (colours, flavours, carriers)
Our international support:
Important network
-
- Work with international / regional / local authorities
- Work with local agents and consulting companies in different countries such as: China / Japan / Brazil / South Africa / Australia etc.
- Work with partners for:
- Development
- Clinical trials
Internal database
Offices in France, in UK and in the USA
Our Added values:
A team with scientific and regulatory expertise: education in nutrition, development of health products, quality and food law, toxicology and human health.
Our philosophy: go beyond the basic regulatory compliance services to assist industry on regulatory aspects for the development of product with health benefits for the consumer, and in compliance with the relevant certifications in dietary, organic and ecological trends.
A full-time team that addresses regulatory issues encountered by our clients in a variety of activities on a daily basis.
Internal database and partners network allowing for large scale of services.
15+ years of expertise.

Our commitment:
- Support our clients through communication with authorities and advocate on regulatory issues in liaison with industry associations.
- Partner with clients on projects from start to finish, including project management.
- Transparency on subcontractor use.
- Breaking down regulatory barriers between countries throughout the world.
- Building a strategy that will facilitate export and international trade / Building bridges between countries to facilitate export and international trade.
And more…
RNI offers training adapted to your international development (regulatory note and summary accompanied by case studies in a selection of countries)