What your strategy for your borderline products ?
For nutrition and health products to be compliant with the regulatory requirements in Europe, the most important step is to classify the product according to the correct regulations. This is not always clear cut and often a product may lie between two or even three categories. The term “Borderline Products” therefore refers to products that may be difficult to classify into a single category due them lying at the edge of several product categories. These products are simply referred to as borderline products until their final status has been decided.
Why is this import?
The choice of productant classification can significantly influence the marketability, distribution, registration procedures, time to market, as well as pre- and post-marketing requirements. For example, food supplement products are relatively simple to place on the market versus a medicine or medical device, so careful consideration on the most appropriate classification is a very important business decision.
How to identify these products?
Criteria which can lead to a product being regarded as a borderline product are generally based on the following:
- Intended purpose / function
What is the aim of the product and for which population groups? For example, medicines and medical devices are for medical purposes while cosmetics and food supplements are not.
- Composition and mechanism of action
Identifying the active ingredients and the dosage required to achieve the intended purpose. Emphasis shall be placed on the mechanism of action at these doses with particular attention to any pharmacological, immunological, and metabolic actions.
- Application site
Different product classifications can have the same application site and methods such as topicals and ingestibles.
- Claims
Health claims for food products are challenging and limited to what is on the authorised list in Europe, while other classifications allow claims to be made provided they are substantiated by the manufacturer.
The decision on a product’s classification must be confirmed on a case-by-case basis, considering all characteristics of the product.
Common Borderline categories
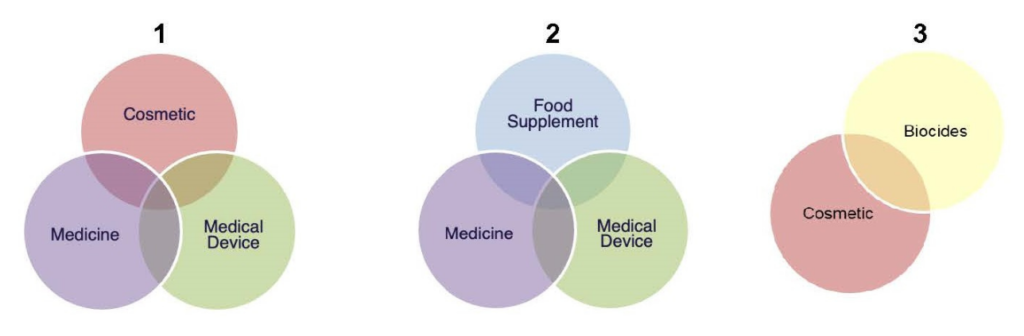 The first group (cosmetic / medicine / medical device) are products that may appear as a cosmetic for topical application but their intended purpose is medical or vice versa. Examples could include: topical gels for massage / joint pain relief, gels for itching, shampoos to prevent hair loss, sunscreens and head lice products.
The first group (cosmetic / medicine / medical device) are products that may appear as a cosmetic for topical application but their intended purpose is medical or vice versa. Examples could include: topical gels for massage / joint pain relief, gels for itching, shampoos to prevent hair loss, sunscreens and head lice products.
The second group (food supplement / medicine / medical device) are products that appear as a food supplement but have a medical purpose or vice versa. Examples could be prebiotics for gastro health, herbal syrups for cough, and substances that influence satiety for managing weight loss and obesity.
The third group (biocide / cosmetic) has been a common borderline group in recent years with the covid outbreak whereby hand sanitisers were incorrectly classified.
Route to market
Another important consideration when dealing with borderline products are the steps involved in getting it to market.
- Premarket: mandatory tests to conduct and the relevant standards (i.e., toxicology, clinical studies), certifications needed (i.e., ISO 13485)
- Registration procedures: involvement of a third party (e.g., notified body), lag time and risk of rejection.
- Marketing: type of product claims, labelling requirements, restrictive populations, and authority oversight.
- Distribution: place of sale and taxes.
- Post-marketing: reporting and vigilance.
Categorising a product can be challenging and any mistakes here could not only bring attention to competent authorities but also limit the success of your product by not considering other marketing opportunities. The European Commission has published several manuals and guidelines addressing borderline issues in a variety of different regulations. There are also many examples of case laws from different member states with real examples for consideration. It is highly recommended to review these documents and stay informed about developments to ensure your product is always classified correctly.
Contact us today to learn more about all our services:



