 If you are developing nutrition products or food supplements with an addition of micronutrients, make sure that you are aware of the latest scientific opinions published by EFSA to ensure a safe and adequate daily amount to achieve the nutritional effect aimed in the future consumer population in your formulas.
If you are developing nutrition products or food supplements with an addition of micronutrients, make sure that you are aware of the latest scientific opinions published by EFSA to ensure a safe and adequate daily amount to achieve the nutritional effect aimed in the future consumer population in your formulas.
During the formulation the formulation, UL (upper limit), MDD (maximal daily dose in food supplements), RNV (reference nutritional value) and intake from several sources (diet…) must be taken into account. EFSA has recently published a new UL (Upper Limit) for vitamin B6 and selenium and a new ADI (Acceptable Daily Intake) for copper.
-
Updated UL for vitamin B6
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a scientific opinion on the UL for vitamin B6. The final opinion was recently published (https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2023.8006). The relationship between an excessive intake of vitamin B6 and the development of peripheral neuropathy is well documented and is considered as the critical effect on which the UL is based.
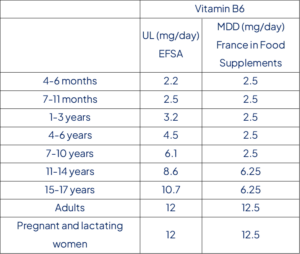
A lowest-observed-effect-level (LOAEL) could not be established based on the available data. A reference point of 50 mg/day is identified by the Panel from a review of scientific literature including data from case reports and vigilance data. With uncertainty factor from clinical safety data and data of a sub-chronic study in dogs, an UL of 12 mg/day is set for adults (including pregnant and lactating women). ULs for children are derived from the UL for adults using allometric scaling; 2.2-2.5 mg/day (4-11 months), 3.2-4.5 mg/day (1-6 years), 6.1-10.7 mg/day (7-17 years). The risk to exceed UL is relatively low in EU populations except for food supplements consumers.
DGCCRF has published recommendations in 2019 for the maximal daily dose (MDD) in food supplements in France for vitamins and minerals (CA_Internet_RS_Nutriments.pdf (economie.gouv.fr)). For Vitamin B6, the MDD is set at 12.5 mg for general population and 2.5 mg for children aged 10 years old and less and 6.25 mg for children aged 10 years old and more. UL and MDD values are summarized in table 1 for vitamin B6. As a reminder, DGCCRF stated in the document that “Vitamin supplementation of infants (0-1 years) and young children (1-3 years) is under medical supervision. Any additional supplementation, without supervision by a health care professional, may pose health risks to this population”.
Table 1: UL and French MDD values for each age range for vitamin B6
-
Updated UL for selenium
Moreover, following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a scientific opinion on the tolerable upper intake level (UL) for selenium (Scientific opinion on the tolerable upper intake level for selenium | EFSA (europa.eu)). Alopecia, as an early observable feature and a well-established adverse effect of excess selenium exposure, is selected as the critical endpoint on which to base a UL for selenium.
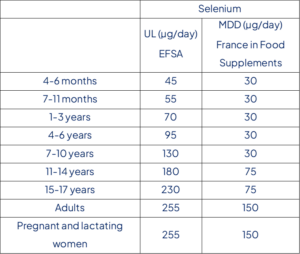
A lowest-observed-adverse-effect-level (LOAEL) of 330 µg/day is identified from a large randomized controlled trial in humans to which an uncertainty factor of 1.3 is applied. A UL of 255 µg/day is established for adult men and women (including pregnant and lactating women). Based on available intake data, adult consumers are unlikely to exceed the UL, except for regular users of food supplements containing high daily doses of selenium or regular consumers of Brazil nuts.
No risk has been reported with the current levels of selenium intake in European countries from food (excluding food supplements) in toddlers and children, and selenium intake arising from the natural content of foods does not raise reasons for concern. Selenium-containing supplements in toddlers and children should be used with caution, based on individual needs.
For selenium, French authority has set up limits for selenium in food supplements: 30µg for children under 10 years old, 75µg for adolescents between 11 and 17 years and 150µg for adults and pregnant/lactating women. Table 2 summarizes the UL and French MDD values for selenium.
Table 2: UL and French MDD values for each age range for selenium
-
Updated ADI for copper
To conclude, EFSA has also published in November 2022 a new assessment of copper exposure intake for the revision of the ADI of Copper (Re‐evaluation of the existing health‐based guidance values for copper and exposure assessment from all sources | EFSA (europa.eu)). The Scientific Committee has concluded that no retention of copper is expected to occur with intake of 5 mg/day and established an ADI of 0.07 mg/kg bw, which is lower than the previous ADI set by EFSA in 2008 (0.15 mg/kg bw). This represents an ADI of 4.2 mg for an adult of 60 kg.
If you want a daily support for R&D and formulation to develop a new safe and efficient nutrition product, food supplement or FSMP (Food for Specific Medical Purpose) taking into account scientific innovation, market trends, regulatory framework, and quality aspects?
Please contact us:
Dr Veronique Traynard, scientific and regulatory consultant: v.traynard@rni-conseil.com
#Selenium #VitaminB6 #Micronutrients #FoodSupplement



