Over the course of the following weeks and months, we will be sharing news that are directly linked to the Covid-19 pandemic and impacting the regulatory affairs markets such as dietary supplements, medical devices, cosmetics, and more.

ADVERSE EVENTS
HOW TO MANAGE REPORTING DURING A PANDEMIC?
THE RNI VIEWPOINT:
If your firm has transitioned to remote work and is still able to maintain the standard operating procedures, RNI recommends continuing to report adverse events as normal. However, if your firm cannot maintain the standard operating procedures for adverse event reporting due to reductions in workforce or office closures, ensure that a company policy is enacted for the pandemic time. This policy should document the information noted by FDA, and should include:
- Documentation of the circumstances affecting continuity of adverse event reporting (e.g. workforce reductions, employee absenteeism, etc.)
- Prioritization procedures based on the FDA guidance
- Documentation of storage procedures for adverse event reports received but not reported during this time
- Standard operating procedure for reporting adverse events following the pandemic to complete within the 6 month time frame per FDAs guidance
>> GET FULL REPORT HERE >>
BE CAREFUL WHEN CLAIMING ON IMMUNITY
THE RNI VIEWPOINT:
RNI recommends that clients exercise extreme caution on any immune claims, refrain from any virus specific claims, and follow standard regulatory requirements. As with all product claims, adequate substantiation is required to support a structure function claim. At this particular time, it is vitally important to scrutinize the substantiation for immune claims and to be extremely conservative in the claims being made for any immune support products. Even very general claims could be seen as misleading claims or as drug claims in the current environment of the COVID-19 pandemic. Additionally, review of the FDA warning letters indicate that the agency is paying very close attention to hashtags in addition to outright claims. Any social media posts should be carefully screened and should refrain from use of COVID-19 related hashtags
>> GET FULL REPORT HERE >>
FULL REPORT – Adverse events: how to manage reporting during a pandemic?
The FDA issued a new guidance in March 2020 about the reporting of adverse events for medical products and dietary supplements in the framework of a pandemic. As the world is currently facing the Covid-19 pandemic, this guidance hence provides recommendations regarding current adverse events to be reported.
Overview
 This guidance has a wide scope, as it includes drugs, biologics, medical devices, combination products, and dietary supplements. Even though the FDA is a regulatory body, the agency details that this guidance does not establish legally enforceable responsibilities, nor is intended to discourage adverse events reporting (AER); adverse events reporting is still mandatory during this time. Industry actors should view it as recommendations in critical times. On a side note, FDA discusses preparedness for a pandemic in ensuring continuity of production by sharing multiple resources: from CDC resources (link here) to HHS recommendations for continuity of operations (link here).
This guidance has a wide scope, as it includes drugs, biologics, medical devices, combination products, and dietary supplements. Even though the FDA is a regulatory body, the agency details that this guidance does not establish legally enforceable responsibilities, nor is intended to discourage adverse events reporting (AER); adverse events reporting is still mandatory during this time. Industry actors should view it as recommendations in critical times. On a side note, FDA discusses preparedness for a pandemic in ensuring continuity of production by sharing multiple resources: from CDC resources (link here) to HHS recommendations for continuity of operations (link here).
During a pandemic, two factors are taken into consideration regarding adverse events reporting: high employee absenteeism (both on FDA and industry side), and an increased use of medical products (related or not to the pandemic). For these reasons, the FDA recommends focusing reporting efforts on three types of reports:
1. Related to medical products indicated for the treatment or prevention of the pathogen causing the pandemic
2. Related to products presenting special concerns from the FDA
3. Other reports indicated in the guidance
Reporting requirements during a pandemic: the FDA may show some “flexibility”
During a pandemic as the one we are currently facing with the Covid-19 pandemic, firms should maintain regular and normal adverse event reporting processes when possible.
However, if a company cannot respect these standard processes, the FDA shows some flexibility for firms who cannot apply standard requirements when it comes to adverse events reporting: “FDA does not intend to object if, because of pandemic-related high employee absenteeism, certain required adverse event reports are not submitted to the FDA within the timeframes required by statute and regulation.” This “flexibility” does not apply to firms that are able to maintain continued operations in reporting adverse events under the standard requirements. In any case, if a company is unable to fill adverse events reporting, it should maintain documentation of high absenteeism justifying the inability to report adverse events, and / or any other factors preventing the company to meet standard requirements.
FDA will be focused on products who regularly report adverse events, especially with newly emerging product-related safety issues, or product problems with associated adverse events.
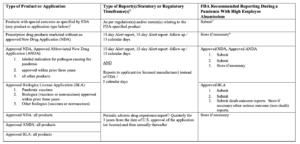
Below is the table explaining the FDA approach to postmarketing safety reporting during a pandemic if normal process of mandatory adverse event reporting is not feasible because of high employee absenteeism.
(1) Additionally, review of the FDA warning letters indicate that the agency is paying very close attention to hashtags in addition to as well as outright claims. Any social media posts should be carefully screened and should refrain from use of COVID-19 related hashtags.
(2) FDA and FTC are jointly monitoring websites and social media for product claims and have established a dedicated task force and website specific to COVID-19 claims. The RNI team has reviewed multiple warning letters issued and it is clear that FDA is taking COVID-19 claims very seriously, and they are on the lookout for companies making drug claims or fraudulent statements.
After the pandemic
If a firm is unable to report adverse events under normal processes during the pandemic, FDA guidance instructs those firms to complete adverse event reporting when the pandemic is over. Firms should follow their plan for the submission of the stored reports not submitted in the regulatory timeframes. “Firms are generally expected to submit stored reports to FDA within 6 months of restoration of the adverse event reporting process to the pre-pandemic state, by prioritizing,” FDA says.
FULL REPORT – Be careful when claiming on immunity
 During the worldwide pandemic period we know today, some actors of the dietary supplement industry might see an opportunity to communicate more about their products, taking risks with immunity or other more general health claims. Companies should be very cautious in making even general immune support claims during this time. GOED, the Global Organization for EPA and DHA, warns us about the general “immunity” claim with Omega-3 and the implication with the coronavirus crisis.
During the worldwide pandemic period we know today, some actors of the dietary supplement industry might see an opportunity to communicate more about their products, taking risks with immunity or other more general health claims. Companies should be very cautious in making even general immune support claims during this time. GOED, the Global Organization for EPA and DHA, warns us about the general “immunity” claim with Omega-3 and the implication with the coronavirus crisis.
Insufficient existing literature = warning
The organization ran a thorough literature search on the topic of Omega 3 and viruses in general. Different keywords were used, such as “virus”, “influenza”, and “omega-3”. 22 positive studies were identified. However, the studies were generally conducted in biased populations; the study populations tended to be considered as a “diseased population” and/or the omega 3 levels of the population were abnormal (high or low). Taking these aspects into consideration, GOED tempers the positive results of these studies relating omega 3 and virus prevention or treatment. “GOED concludes that there is an insufficient body of scientific literature to connect omega 3 to benefits of either positive general or viral immunity outcomes in a healthy population,” a GOED representative says.
Regulatory bodies such as the US Food and Drug Administration (FDA) and Federal Trade Commission (FTC) ensure that they are monitoring the situation, especially when it comes to immunity, immune system and virus treatments. The increased scrutiny is evident based on the number of warning letters FDA has issued in March to companies making COVID-19 related claims. FDA and FTC are jointly monitoring websites and social media for product claims and have established a dedicated task force and website specific to COVID-19 claim. The RNI team has reviewed multiple warning letters issued and it is clear that FDA is taking COVID-19 claims very seriously, and on the lookout for companies making drug claims or fraudulent statements.